Hydrogen-Mediated C-C Bond Formation

From a mechanistic point of view, the key for the reductive aldol 1 2 3 4 and mannich reaction 5 they reported is how to tune the properties of the metal hydride to just coordinate to the alkene and form the metal-enolate which then undergo an aldol reaction with the aldehyde and not to reduce the aldehyde and C-C double bond. The catalyst chosen and the base they added is crucial, which can help form a monohydride species and disable the dihydride cycle which will reduce the C-C double bond.

For the Alkyne-Carbonyl 6 7 and Alkyne-Imine 8 9 coupling reaction, however, an acid additive is necessary. Based on a computational study by Musashi and Sakaki and their experimental evidence they believe that the acid can help form a six member ring intermediate which require lower energy than the four member ring hence accelerate the reaction

Interestingly, in order to probe the role of acid they used a chiral bronsted acid together with a achiral ligand to do the reaction and found that for the pyridinealdehyde 82% enantioselectivity could be obtained. It's pity that they did not explore this kind of strategy which might leading to some new chemistry. They should be the first to combine the chiral phosphoric acid with achiral metal (If Reuping's paper adopt the same kind of idea, it will be not original).

For the substrate bearing a pyridine ring, they proposed another mechanism in which the acid interact with the basic pyridine nitrogen and lower the LUMO of the substrate to facilitate oxidative coupling.
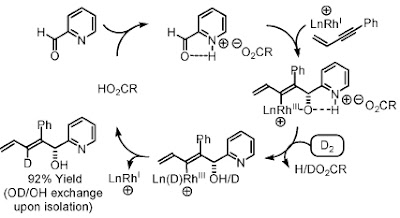
What I learned from this chemistry is that the mechanism is vital to catalysis. Novel ideas could be obtained only when you have a very good command of all kinds of mechanisms. Also you should have the ability, courage and perseverance to realize the ideas.

2 comments:
For first combination of metal catalysis and organocatalysis, see: “Catalytic Enone Allylation via Concomitant Activation of Latent Nucleophilic and Electrophilic Partners: Merging Organic and Transition Metal Catalysis” Jellerichs, B. G.; Kong, J.-R.; Krische, M. J. J. Am. Chem. Soc. 2003, 125, 7758.
Thanks for your comment. I have seen this paper after I write this post.I have to say Prof. Krische have done a lot of excellent work in catalysis.
Post a Comment