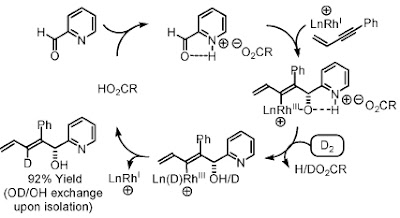
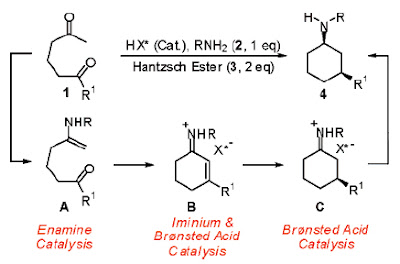
Cascade Reaction
Later, Terada using their chiral phophoric acid catalyzed a tandem aza-ene/cascade cyclization reaction.2 The chiral phosphoric acid can catalyze two aza-ene reaction sequencely to generate two chiral center. Then the intramolecular cyclyzation can produce the third chiral center induced by the substrate chirality. Though not so elegant as List's work, the efficiency of the reaction is very satisfactory, with only 2 mol % of catalyst loading the reaction can finish in 30 mins.

Very recently, Wang reported their very comprehensive work on the asymmetric cascade Michael-Alkylation reaction.3 This is another well designed cascade reaction. The key for the reaction is the selection of a suitable substrate which can act both as a nucleophile for the Michael Addition step and electrophile for the subsequent Alkylation step. Bromo-malonate seems to be the best of the choice. The reaction starts with a iminium catalyzed Michael Addition and followed by a enamine catalyzed alpha-alkylation, affording the cyclopropane, a very important and yet difficult to generate product, in very high yield, diastereoselectivity and enantioselectivity. Fantastic, isn't it?

After posting this post, I found an article in Chemistrys wrold titled " At the top of the cascade". It's really a good article on organocatalysis and as you can see in the article, MacMillan used the term " Organocascade". I believe in the near future there will be a pile of papers on the so called "Organocascade" reactions. Organocatalysis is beginning to change from improving the exsiting reactions to create something new. Though I have quit this hot field, I just feel relieved. The competition in this field might drive you crazy.